The Apple Watch has grown in versatility since it was first introduced by the Cupertino-based company. It has proved to be a lifesaver in several instances due to its impressive portfolio of features. The latest news is that the Apple Watch can now be used in AFib clinical studies, thanks to a new FDA approval. This is cherry news for Apple after the issues related to pulse oximetry patent rights that affected the Watch Series 9 and Watch Ultra 2.

The United States Food and Drug Administration (FDA) has approved the atrial fibrillation (AFib) detection software on the Apple Watch and this can be deployed for clinical trials. The Apple Watch’s AFib history feature is the first digital health technology that has scaled through the FDA’s Medical Device Development Tools (MDDT) program. Atrial fibrillation is an abnormal heartbeat condition that is also known as arrhythmia. The AFib history feature is designed to observe a subject’s AFib burden weekly estimate before and after using a cardiac ablation device during a clinical trial.
The Apple Watch AFib History feature regularly checks the user’s heart rhythm for signs of AFib. After a week of checking, it estimates how often your heart was beating irregularly while wearing the Apple Watch. The AFib detection software is also designed to be used as a biomarker test to help evaluate estimates of AFib burden as a secondary effectiveness endpoint in clinical studies to evaluate the safety and effectiveness of cardiac ablation devices. These are important emerging devices that could significantly reduce the burden of the atrial fibrillation condition on a patient.
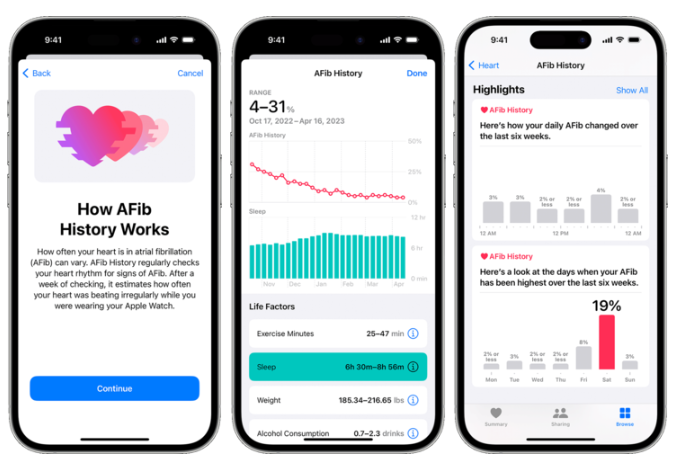
It is noted that atrial fibrillation can be detected by several traditional methods including using the ECG or even a home blood pressure monitor. There are also some smart wearable devices apart from the Apple Watch that are capable of detecting atrial fibrillation.
RELATED:
- LG CineBeam Q Projector With Portability, 4K Resolution Launched In India
- LG OLED evo C4 Gaming TV goes on sale in China less than three months after its announcement
- Huawei Pura 70 Ultra Retractable Camera Explained
- Samsung G60SD gaming monitor with 360Hz 27″ 2K OLED panel launched in China for $989
- Xiaomi Redmi Monitor A27Q 2025 with 27-inch 2K 100Hz panel now up for sale in China







