The known wearables and smart products maker, Fitbit, has its upcoming ventilator receive an emergency approval from the FDA. The US government’s Food and Drug Administration has given Fitbit an Emergency Use Authorization for its Flow ventilators, which will be used to help patients with COVID-19 infection.
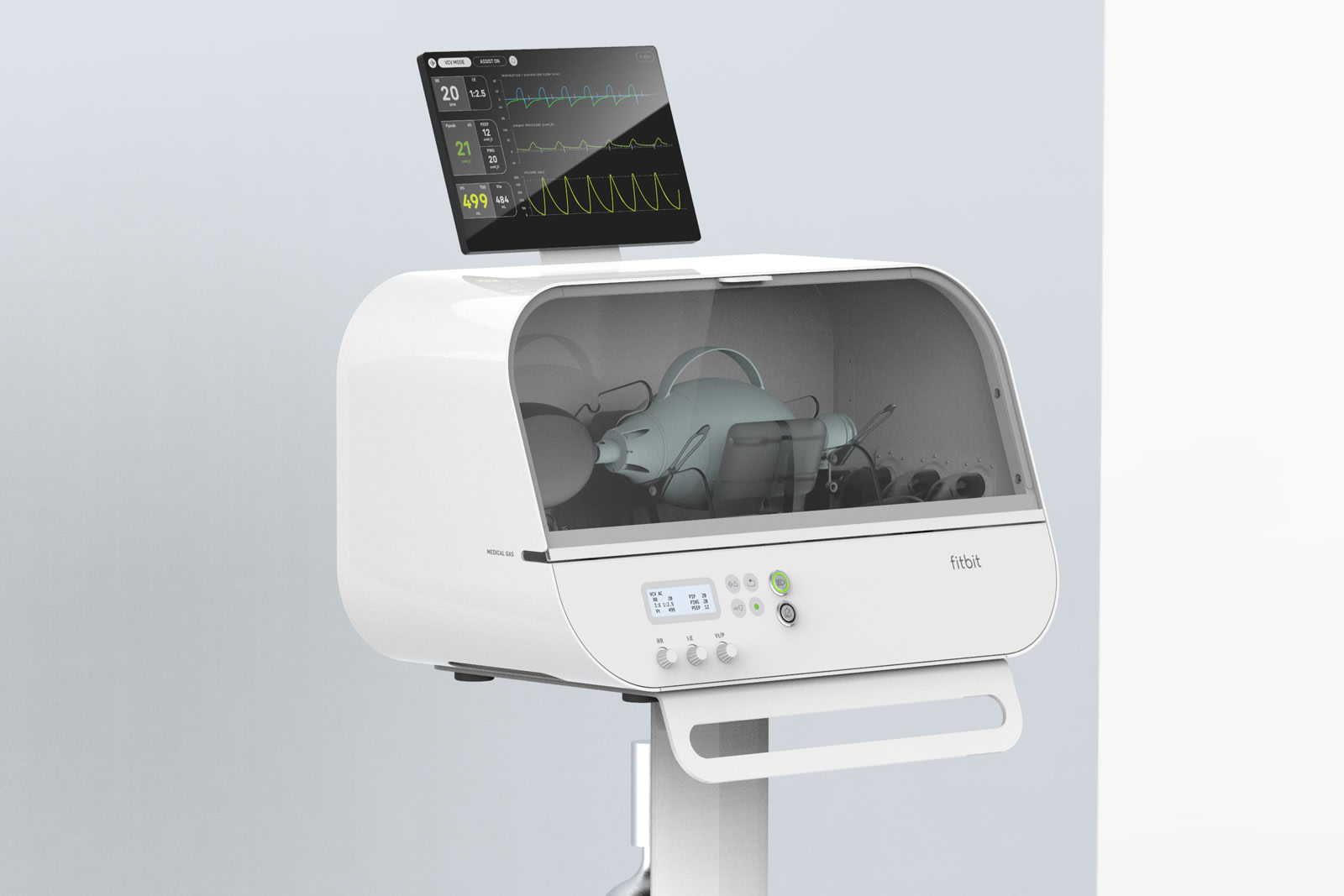
At the moment, the US government is reportedly “in talks” with Fitbit to supply its Flow ventilator units. For those unaware, Flow is a ventilator built with advanced sensors and alert systems that features a simple user interface and is easy to operate. This would eliminate the need for specialized training and is also built to target “lower price range” or cost effective emergency ventilators.
Editor’s Pick: Lenovo Legion gaming phone’s Geekbench listing appear; Likely to debut this month
Fitbit is confident in its production capabilities, stating that it will have an advantage in activity tracking since it is a known smart product manufacturer. However, the company still has a few hurdles to tackle, with the primary one being a customer base. It needs to find customers to sell to since its arrival to the market is relatively late. Furthermore, the demand for this ventilator is also uncertain as another wave of COVID-19 occurring is unknown as well. As it stands right now, the Flow could be crucial in key areas, but only if the situation demands it.
UP NEXT: Xiaomi Youpin launches the Haylou LS04 Solar Smartwatch, features a 30 day standby and 12 sports modes
(Via)







