A breakthrough for diabetes management arrived on March 6th with the FDA‘s approval of Dexcom’s Stelo glucose biosensor system. This revolutionary device, the first of its kind, allows for over-the-counter, prescription-free blood sugar monitoring for individuals with non-insulin-dependent diabetes or those at risk of developing the condition.
The glucose monitor displays readings every 15 minutes
Unlike traditional methods, Stelo utilizes a wearable sensor and smartphone app to continuously monitor glucose levels, displaying readings every 15 minutes. Each sensor lasts up to 15 days before needing replacement, offering a convenient and long-lasting solution.
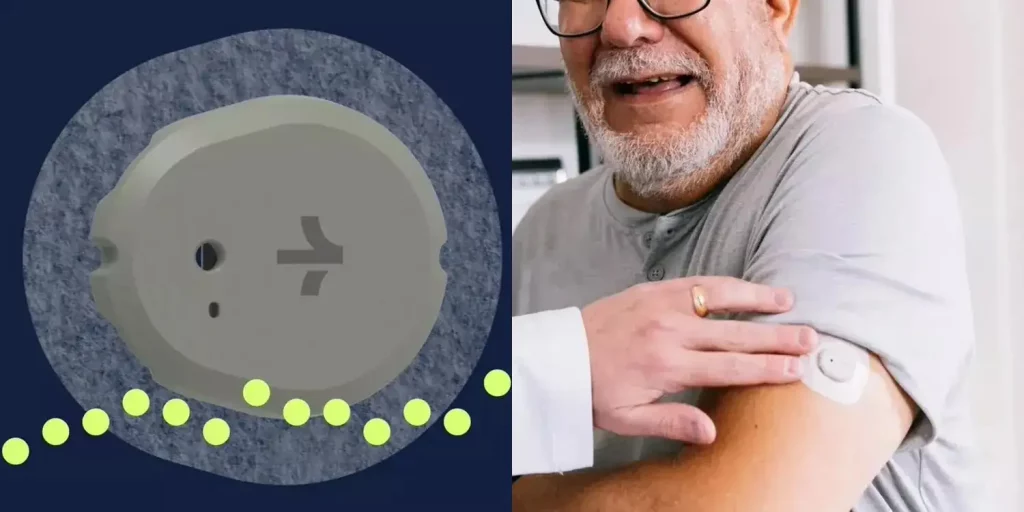
The FDA highlighted the significance of this approval, emphasizing its potential to increase accessibility to blood sugar monitoring and empower individuals to manage their health from the comfort of their homes.
While Stelo represents a major step forward, the ultimate goal in diabetes management remains non-invasive blood glucose monitoring. This technology, though initially planned for the Apple Watch, has yet to be realized due to current limitations. The FDA also cautions against unapproved non-invasive alternatives, stressing their unreliability.
Despite the current absence of non-invasive options, Dexcom’s Stelo marks a significant advancement in diabetes management, offering a convenient and accessible solution for many people. The future of blood sugar monitoring, however, holds the promise of even more innovative and non-invasive approaches.
RELATED:
- Samsung Galaxy Watch receives FDA approval for sleep apnea detection
- Neuralink Receives Approval from FDA for Human Testing
- Get latest Oneplus 12 Phone for $699 on Geekwills
- Get $100 OFF on Xiaomi 14 Pro at Giztop (1TB Variant)
- Lenovo Legion Y700 2023: Save $100 on this 8-inch gaming Android tablet
- How to add/remove someone in a WhatsApp group (with screenshots)
(Via)







